Sponsors
-
Featured Companies See all (42)
-
Featured Conditions See all (32)
-
Bloating
Abdominal distension -
Colitis
Ulcerative colitis -
Post-Cholecystectomy
Post-Gallbladder Rem -
Enlarged Prostate
Benign Prostatic Hyp -
Angular cheilitis
Cheilosis -
Leg cramps during pr
Pregnancy-Induced le -
Alcoholism
Alcohol abuse -
COVID-19
Coronavirus -
Trichomoniasis
Trichomonas vaginali
-
Featured Nutraceuticals See all (50)
-
S. Boulardii
Saccharomyces Boular -
Chromium
Cr -
Vitamin B7
Biotin -
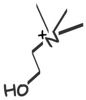
Choline
Phosphatidyl Choline -
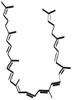
Lycopene
Ψ, Ψ -
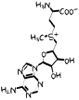
SAM-e
S-adenosyl methionin -
Papain
Papaya proteinase I -
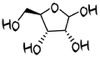
Ribose
D-ribose
-
-
Featured Herbals See all (45)
-
Hydrangea " Sev
Hydrangea arborescen -
Gravel root; Joe pye
Eupatorium purpureum -
Valerian
Valeriana officinali -
New Jersey Tea
Ceanothus americanus -
Cramp Bark; Black Ha
Viburnum opulus -
Chinese wormwood or
Artemisia annua -
Senna
Cassia senna -
Turmeric
Curcuma longa -
Goat's Rue
Galega officinalis -
Mullein
Verbascum thapsus
-
-
Featured Actions See all (50)
-
Thermogenic
n/a -
Sedative
Nervine -
Antihemorrhagic
Hemostatic -
Decongestant
n/a -
Cholagogue
Bile stimulant -
Cough suppressant
Antitussive -
Demulcent
n/a -
Aphrodisiac
Sexual tonic -
Antipyretic
Febrifuge
-