Sponsors
-
Featured Companies See all (42)
-
Featured Conditions See all (28)
-
Gingivitis
Inflamed gums, g -
Ferretin, Elevat
Hyperferritinemia -
Dry Eyes
Keratoconjunctivitis -
Blastocystis hominis
Blastocystis hominis -
Giardia
Giardiasis -
Malaria
Quartan malaria, -
Colitis
Ulcerative colitis -
COVID-19
Coronavirus -
PMS
Premenstrual syndrom
-
Featured Nutraceuticals See all (48)
-
Taurine
Amino sulfonic acid -
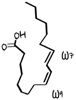
Omega 3-fatty acids
Omega-3s EFA -
B. longum ssp. Infan
Bifidobacterium long -
Molybdenum
Mo -
DHA
Docosahexaenoic acid -
S. Boulardii
Saccharomyces Boular -
Chromium
Cr
-
Featured Herbals See all (44)
-
Witch hazel
Hamamelis virginiana -
American Ginseng; Ca
Panax quinquefolius -
Chamomile (Germa
Matricaria recutita -
Ephedra " Ma Hu
Ephedra sinica -
Castor oil
Ricinus communis -
Turmeric
Curcuma longa -
Goat's Rue
Galega officinalis -
Fennel
Foeniculum vulgare -
Lemon Balm
Melissa officinalis -
Butcher's Broom
Ruscus aculeatus
-
-
Featured Actions See all (50)
-
Nervine
n/a -
Cough suppressant
Antitussive -
Antiparasitic
Paracidal -
Antibacterial
Bactericidal -
Hormone Regulator
Hormone Balancer -
Expectorant
n/a -
Diuretic
n/a
-